Research Report
Effects of Vitamin A Supplementation in Female Broodstock Diets of African Catfish on Egg Quality, Egg and Liver Retinol, Ovary Estradiol, and Larval Survival 
2 Imefon Udo Udo - Department of Fisheries and Aquatic Environmental Management, University of Uyo, Uyo, 520103, Akwa Ibom State Nigeria
3 Ofonime Edet Afia-Department of Fisheries and Aquatic Environmental Management, University of Uyo, Uyo, 520103, Akwa Ibom State Nigeria
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2024, Vol. 14, No. 5 doi: 10.5376/ija.2024.14.0027
Received: 20 Aug., 2024 Accepted: 25 Sep., 2024 Published: 25 Oct., 2024
Okure G.P., Udo I.U., and Afia O.E., 2024, The effects of vitamin a supplementation in female broodstock diets of African catfish on egg quality, egg and liver retinol, ovary estradiol, and larval survival, International Journal of Aquaculture, 14(5): 266-279 (doi: 10.5376/ija.2024.14.0027)
The effect of feeding five experimental diets NoRE, 1666RE, 3332RE, 6664RE, 13,328RE containing different vitamin A acetate levels (VA): 0, 1666, 3332, 6664 13328 40, 60 IU kg-1diet, respectively, on egg quality, egg and liver retinol, ovary estradiol, and larval survival rate of African catfish (Clarias gariepinus) broodstock was studied in a tripodal 60-day feeding trials. These were designated as F0, F1 and F2, making a 180-day feeding experiment. The preliminary phase (F0) was conducted to detoxify the broodstocks of cellular VA. The second and third phases (F1, F0) were conducted to access first and the second spawning seasons. During the preliminary phase experimental fish were fed with the basal diet (control diet) thrice a day three percent (3%) fresh body weight. The mean percentage fertilized egg was significantly higher in fish fed diets 6664 RE and 13328 RE than others and ranged from 73 to 98% for both halves. The mean percentage hatched egg was significantly higher (p<0.05) in fish fed 13,328 IU Kg-1 than others and ranged from 80 to 100% for both halves. The retinol content in eggs was significantly higher in fish fed 13,328 IU Kg-1 than others and ranged from 175 to 3884 and 204 and 4022 IU kg-1 for F1(60) and F2(60) respectively. No retinol was detected in livers. The Estradiol residues significantly (p<0.05) increased with increase concentration and was significantly higher (p<0.05) in F2 (60). Larval survival rate was significantly higher (P<0.05) in fish fed diet 3332 to 13328 RE and was significantly higher in F2 (60) than F1 (60). Retarded growth, poor feed efficiency, abnormal coloration, and bone and fin erosion were observed as signs of VA deficiency in fish fed 0 IU kg−1. No effect of VA excess was observed. On the whole, fortification of brood stock fish diet of C. gariepinus has resulted in positive growth and reproduction parameters. The aquaculture industry should follow the lead of the more advanced poultry industry and begin formulating broodstock and sex-specific rations. Vitamin A dosage as high as 13,328 IU kg-1 diet is recommended for such ration.
1 Introduction
The first step in ensuring a successful aquaculture venture is selecting appropriate broodstock. According to Chattopadhyay (2017), this process involves identifying individuals with desirable phenotypes for a particular trait and using them as future broodstock to produce offspring with similar traits. Broodstock can be sourced from domestic or wild ponds. Nguyen et al. (2013) found that 78.1% of hatchery broodstocks were from domestic ponds, 6.3% were from wild ponds, and 15.4% were from both. Broodstock can be obtained from various sources, including grow-outs, export-oriented grow-outs, capture fishing, own hatchers, or other hatchers, in order of importance (Belton et al., 2010). The use of pond-reared broodstock is becoming increasingly popular in aquaculture.
The unpredictability and variability of reproductive performance of the susceptible species is a significant obstacle to the successful mass-production of juveniles (Segura et al., 2021). In order to guarantee the highest quality of juveniles available for stocking, it is important to comprehend and utilize the biological (in particular reproductive) characteristics of the stock. This includes nutritional, health, and genetic characteristics, as well as appropriate husbandry practices to ensure the offspring have the reproductive and genetic characteristics they need.
Nutrient levels in broodstock diets have been the subject of much attention in the past two decades. Nutrient levels in broodstock feeds can affect gonadal maturation, fecundity, fertilization, embryo development, and larval quality. It has been demonstrated that improved nutrition and feeding can significantly improve egg quality, sperm quality, and seed production. Dietary nutrient level has a marked effect on fertility. For example, there is a positive correlation between dietary eicosapentaenoic acid (EPA) and arachidonic acid (AA) levels and fertilization rates in gilthead sea bream broodstock (Izquierdo, 2001). There is also a positive effect when supplemental dietary vitamins are used (Furuita, 2001; 2003) including vitamins E and C and carotenoids.
Several nutrients, including proteins, essential fatty acids, vitamins, and carotenoids can significantly affect embryo development by improving egg morphology and hatching rates. A well-balanced, essential amino acid profile can improve vitellogenin synthesis (Alagawany et al., 2020). High dietary omega-3 HUFA levels improve the percentage of morphologically normal eggs, and improve egg quality and viability. Carotenoids improve egg quality, larval survival, and development. Several studies have shown the effect of broodstock nutrition on seed or larval quality. For example, increasing dietary lipid levels in broodstock diets can result in the production of large newly hatched larvae with an increased survival rate (Tacon, 2000). The quality of juveniles produced by hatcheries is dependent on a range of factors, including the sourcing of the stock, harvesting of nursery ponds, and other factors.
Broodstock nutrition has been identified as one of the most under-explored and under-invested areas of aquaculture research (Berlinsky et al., 2020). Research on broodstock nutrition is limited and relatively costly, making it a high-risk area of research. Furthermore, the feeding period required for broodstock to influence egg composition is variable, making it difficult to conduct long-term experiments. Furthermore, broodstock nutritional studies are complicated by fish reproductive strategies, such as pelagic eggs producing a large number of small eggs, while demersal spawner eggs produce a large number of larger and therefore fewer eggs. Most carnivorous breeding programs select broodstock based on their performance in fishmeal-based diets, however, future plant-based diets could potentially affect fish breeding programs should genotype and diet interactions arise. Maternal nutrition is a key factor in the success of larvae. The diet of the broodstock affects the levels of fatty acids, amino acids, and vitamins present in the egg and its progeny (Mejri, 2021). Therefore, the diets of broodstock should be formulated in such a way as to guarantee that all nutritional requirements of the fish species under cultivation are fulfilled.
Vitamins are natural compounds that are necessary for the development, reproduction, and health of fish and should be included in the diet in moderate amounts (NRC, 2011). Vitamin deficiency is the most frequent cause of deficiency in the commercial aquaculture sector (Webster and Lim, 2001). Symptoms of vitamin deficiency in fish include abnormal swimming, dermatitis, bone deformity, edema, ocular and gular pathology, hemorrhage, liver disease, and growth retardation. The most important vitamins to include in fish diets are A and D, E and K (which are fat-soluble), as well as vitamin C and the B complex (which are water-soluble).
Vitamin A is essential for vision, immune function, fertility, and overall health of vertebrate animals. In fish, high dietary intakes of vitamin A have been demonstrated to improve certain immune functions, including the migration of kidney leucocytes and serum bacteriostatic activity in Atlantic Salmon Salmo salar (Berntssen et al., 2016), serum complement, antiprotease activity, leukocyte migration, serum complement activity and phagocytic activity in Rainbow trout Oncorhynchus mykiss (Amar et al., 2004), respiratory burst activity in gilthead seabream Sparus aurata (Cuesta et al., 2002) and serum anti bacteriostatic activity in Japanese Flounder Pralichthys olivaceus (Hernandez et al., 2007). In broodstock, high fecundity and early growth of offspring were recorded in Rainbow trout Oncorhynchus mykiss (Fontagné-Dicharry, 2010), positive effects on fecundity and larval quality in Penaeus chinensis (Mengging et al., 2004), high maturation rate (60%), spawning rate (36%) and early occurrence of first spawning (14 days after eyestalk ablation) in Penaeus monodon. The nutritional content of broodstock feeds has a significant impact on various reproductive processes, including: Gonadal development, egg production (fecundity), successful fertilization, healthy embryo formation and quality of larvae. In essence, proper nutrition in broodstock feeds is crucial for optimal reproductive performance and offspring quality. The African catfish is one of the most widely cultivated species in the world. This fish species cannot synthesize VA and most be obtained from the diet. However, there is paucity of information on the impact of dietary vitamin A levels on Estradiol (E2) concentration, fertilization rate, hatching rate, and fry survival rate of C. gariepinus. Therefore, this study aimed to assess the impact of different dietary vitamin A content levels on Egg E2 content, fertilization rate, and hatching rate, as well as on the fry survival rate.
2 Materials and Methods
2.1 Study area
This study was conducted at the Department of Fisheries and Aquatic Environmental Management, Fish Farm Hatchery Complex, University of Uyo, geographically located at latitude of 5.0408 °N and longitude 7.9198 °E.
2.2 Experimental diet formulation and production
2.2.1 Collection and processing of feed ingredients
Feed ingredients used in this experiment include soybeans, fishmeal (Ethmalosa fimbriata), palm kernel cake and white maize which were procured from Uyo main market. The ingredients were brought to the processing unit of the University of Uyo, fish farm complex where they were finally processed as follows: Soybean was toasted until it becomes brown and the chaff was blown out before grinding into powdery form. Fishmeal, white maize and palm kernel cake were ground into homogenous fine particles.
2.2.2 Determination of biochemical composition
Total moisture and ash content of the ingredients and experimental diet were determined according to the procedures of Shabir et al. (2018). Crude protein content was estimated using the Kjeldahl method as outlined by Wybraniec et al. (2013) and Maehre et al. (2018). The crude fat or ether extract (EE) procedure estimates the quantity of lipids and was performed following the guidelines of Malik and Mandal (2022). To perform the procedure, 10 grammes of dried samples were ground and extracted with an n-hexane for 4 hours and the remaining residue is dried and weighed. Ether extract was calculated as the difference between the original sample and the ether extract residue. Crude fibre was estimated using the procedure outlined by Madhu et al. (2017). The calculation for nitrogen free extract was done using the formula:
.png)
The total phosphorus was determined by a spectrophotometric–colorimetric method as outlined by Gliszczyńska-Świgło and Rybicka, (2021). Calcium was determined by spectrophotometric determination of with Dibromo-p-methylsulfonyl as outlined by Li and Zhai (2020). Chromatographic determination of lysine and methionine as outlined by Pundir et al. (2021) was adopted. The digestible energy content of ingredients and experimental diets was predicted from their proximate composition (Table 1), according to the equation of Núñez-Sánchez et al. (2019) and the modified equation of Zeyner and Kienzle (2002), as presented in Kienzle and Zeyner (2010) as follows:
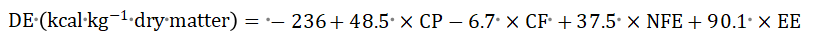
.png) Table 1 Proximate composition and digestible energy of the feed ingredients used in this experiment Table caption: FM = fishmeal; SBM = soybean meal; WMM = white maize meal; PKC = palm kernel cake DM = dry matter CP = crude protein; CF = crude fibre; EE = ether extract; P = phosphorus; Ca = calcium; NFE = nitrogen free extract; LS = lysine; MT = methionine; DE = digestible energy |
2.3 Experimental diet formulation
The diets for this experiment were formulated using feed formulation software for windows (Winfeed 2.8) which formulates feed by linear programming technique (LPT). All diets were formulated on dry matter basis using the proximate compositions of the feed ingredients according to Udo et al. (2011) (Table 2).
.png) Table 2 Ingredients and nutrient compositions of the experimental diets Table caption: 1: Fresh (PAMOL, Calabar, Nigeria);2: toasted, (Uyo, Nigeria);3: locally made from Ethmalosa fimbriata (Uyo, Nigeria);4: milled (Uyo, Nigeria);5: menhaden6iodized (Dangote, Nigeria);¥calculated digestible energy, DE (MJ kg-1DM) = [(CP x 4) + (EE x 9) + (TC x 4)] x 0.042; Vitamin A (Korea United Pharm. Inc. (404-10, NojangRi, Jeondong-Myeon, Yeongi-Kun, Chongnam Korea) |
2.3.1 Feed milling and drying
Diets formulated in percentage were then converted to weight basis. The ingredients were measured using Camry kitchen weighing balance into a mixer where they were blended thoroughly. Five percent of cassava starch was used as a binder. Hot water was then added into the mixture and mixing continued. After 10 minutes, the dough was pelleted using manual pelletizer of 6 mm die ring and dried thoroughly. Synthetic vitamin A, dissolved in liquid hexane at 100 mg: 0.25 liter of liquid hexane was sprayed on the pellets according to different treatments as follows: Treatment 1 (T1), 2 (T2), 3 (T3), 4 (T4) and 5 (T5) received 0IU kg-1, 3332IU kg-1, 6664IU kg-1 and 13,328 IU kg-1. Retinol and were designated as NoRE (no retinol), 3332RE, 6664RE and 13328RE respectively. The experimental diets were then air-dried for 15 minutes and stored in black polythene bags prior to feeding.
2.4 Experimental procedures
2.4.1 Experimental design
This study was conducted for 6 months using fifteen concrete tanks (rectangular) of 18.11 m3 volume with water depth and volume of 2.1 m and 14.41 m3 respectively. The tanks were arranged in triplicate and designated as A1, A2, A3, B1, B2, B3, C1, C2, C3, D1, D2, D3and E1, E2, E3, giving a completely randomized design (CRD) of five (5) treatments and three (3) replicates.
2.4.2 Stocking
The fifteen tanks were stocked with 300 adult females of C. gariepinus (20 per tank) of 1.60±0.01 kg mean weight collected from the Hatchery Complex of the University of Uyo fish farm. Three hundred (300) male C. gariepinus were also reared in 15 other tanks for breeding experiment. Prior to stocking, the fish were acclimated for 7 days during which feeding was done ad libitum twice daily using 6 mm pellets of commercial feed (ECOFLOAT®) with the following nutrient composition: Crude protein 34%, Crude fat 6%, Ash 7%, Crude fibre 3%, moisture 8%, Calcium 1.5, Phosphorus 0.5% and sodium 0.3. Prior to the commencement of the trial, the experimental fish were starved for 24 hours after which the average initial biometric parameters in each experimental unit including the wet body weight were measured. The wet body weight was taken to the nearest gram. The adult females were then distributed into five nutritional treatments, which differed by the amount of VA supplemented in the diet: T1 = 0, T2 = 1666, T3 = 3332, T4 = 6664, and T5 = 13,328 IU kg-1of VA.
2.4.3 Experimental fish management
The experimental diets were fed to fish according to Treatments at three percent (3%) of their fresh body weight thrice daily at 8:00, 12:00 and 18:00 hours. Feeding for the first 60 days was done with the basal diet (control diet) which was poor in VA to reduce body VA content and to ensure that the fish were well matured. Afterward, they were fed for 60 days (1st half) and another 60 days (2nd half) with the experimental diets at the same interval as of the first 60 days.
2.5 Data collection
At the end of feeding trial, a total of 150 gravid females were collected from the five experimental treatments (30 from each Treatment) and the whole ovaries, and liver were excised, Samples of the eggs and livers were taken to the labouratory for analyses of VA content. 17β-estradiol (E2) levels were measured after the first and second half 60days.
2.5.1 Determination of percentage fertilization rate
Male and female donors of C. gariepinus for breeding experiment were obtained from the remaining one hundred and fifty (150) brood fish. The donor fish selected by visual conditions, namely: the female with the size of the stomach that marked with fat and tender stomach, whereas in males it is characterized by the presence of swollen urogenital papillae.
2.5.2 Fish breeding
Day 1 Activities: The breeding experiment was done in the 15 tanks of the Hatchery unit according to the experimental treatments and replicates. The tanks were washed and dabbed with formalin – soaked foam to disinfect and reduce algal bloom. An empty bowl was kept on top of the weighing balance and zeroed. This was done to measure the weight of the brood stocks to be used and quantity of hormone to be injected. Female brood stocks were taken out of the tank into the bowl and weighed. The final weights were 2.72 kg, 3.13 kg, 4.31 kg, 3.40 kg and 5.58 kg for T1, T2, T3, T4 and T5 respectively. The brood stocks were then injected with ovulin hormone at 0.5 mg kg-1 of their body weight at angle 45o intramuscularly. The dosages were calculated (Table 3).
.png) Table 3 Calculated dosage of Ovulin injected to the Female Brood stock at varying intervals during the breeding experiment |
After injection, the body of the fish were massaged to allow easy distribution of the hormone in the fish’s bodies and to avoid swelling, and the hormone from oozing out. The injected female stocks were then placed in their tank and the male in their separate tanks. Temperature in the tank was maintained at 27 ℃. Latency period of 12 hours was observed from 9.00 pm~9.00 am.
Day 2 Activities: Male broodstocks were brought out and placed on a leaf, sacrificed and excised for the gonads (two testes from each male broodstock). The blood was washed out from the gonads with saline solution. A new razor blade was used to cut the testes open to get the milt into a clean bowl. The injected female brood stocks were brought out carefully from the tanks. A moist towel was used to hold the head of the female broodstocks gently for stripping. The eggs were then stripped into the bowls of milt gently by pressing the abdomen. Stripping was stopped whenever there was any blood stain. The matured eggs were amber in colour. The milts and eggs were mixed thoroughly with a plastic spoon. Milt was diluted with physiological NaCl (0.9%). The ratio of milt and diluents was 1:9.
Fertilization Process: Two hundred (200) eggs from each experimental treatment were inserted in a plastic bowl, mixed with 1 ml of fish sperm (taken from the reared males) measured with a plastic syringe added to fertilize the eggs in each replicate of the treatments. Fertilizer solution (H2O) was then added and stirred for 2 minutes until flattened in order to fertilize. The eggs were then spread on the hatching strain then hatched on the hatching tub. The number of fertilized and unfertilized eggs was counted under a microscope (x 40 magnification). The fertility rate was determined by observing the sample (± 200 eggs). The fertilization rate was calculated according to Adebayo (2006) as follows:
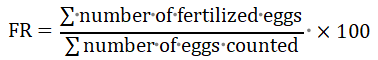
Note: The fertility rate was calculated after the eggs fertilized for 9~10 hours. The fertilized egg was marked with a clear and transparent color, whereas the unfertilized egg was characterized by its feculent white color due to the breaking of the egg yolk. Fertilization rate was determined by calculating the total number of eggs removed and counting the number of eggs that were clear or feculent white. Then, it was included in the calculation formula of fertilization level to know the percentage.
Hatching rate was calculated according to Adebayo (2006) as follows:
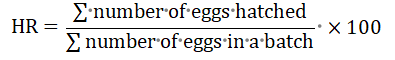
Note: This hatching rate was calculated after 9 -10 hours of hatching eggs by calculating the number of hatching larvae or the number of non-hatching eggs.
2.6 Vitamin A concentration analysis
The Vitamin A concentration in the eggs and liver were analyzed after 60 days using photometric procedures according to Kumar et al. (2021).
2.7 Determination of estradiol
Measurement of Estradiol (E2) was done by the use of gas chromatography on micro columns of Sephadex LH-20 with benzene: methanol (85:15) according to Zou et al. (2007).
2.8 Water quality monitoring
Ammonia, pH, dissolved oxygen (DO), and temperature were measured on weekly basis using standard meters and kits. Ammonia was measured calorimetrically using ammonia test kit, pH was measured using Hanna pHep pocket-sized pH meter, DO was measured using DO meter while Temperature was measured using mercury-in-glass thermometer.
2.9 Statistical analysis
Data obtained will be subjected to one-way analysis of variance (ANOVA). The means from the various treatments will be compared for significant differences (p<0.05), using Duncan’s multiple range test with the aid of IBM SPSS Version 21 for windows.
3 Results
3.1 Effect of vitamin a supplementation on the water quality parameters
The water quality parameters are fluctuated slightly (Table 4).
.png) Table 4 Water quality parameters (mean±SD) during the 6 months culture period Table caption: NH3 = ammonia; DO = Dissolved oxygen; M. Temp. = Morning Temperature; Opt.= Optimum |
3.2 Effect of supplementation of vitamin a on egg quality parameters
3.2.1 Effect of supplementation of vitamin A on egg fertilization rate of African Catfish (C. gariepinus) Broodstock
The effects of Supplementation of Vitamin A on egg fertilization rate of African Catfish (C. gariepinus) broodstock is presented (Table 5) and the difference between F1 (60) and F2 (60) (Figure 1). The mean percentage fertilized egg was significantly higher in Treatment 4 and 5 than others and ranged from 73 to 98% for both halves. Egg fertilization rate in F2 (60) was significantly higher (p<0.05) than F1 (60) (Table 5).
.png) Figure 1 Mean percentage fertilized egg of Clarias gariepinus (Error bar = SEM) |
.png) Table 5 Means of the reproductive parameters of female Clarias gariepinus fed diets with varying levels of vitamin A (n = 60 per treatment) Table caption: Values are mean ± standard deviation. Values with the same superscript letters are not significantly different. Ns=not significant |
3.2.2 Effect of supplementation of vitamin A on egg hatchability rate of African Catfish (C. gariepinus) Brood stock
Effect of Supplementation of Vitamin A on egg hatchability rate of African Catfish (C. gariepinus) Broodstock is presented (Table 5) and the difference between F1 (60) and F2 (60) (Figure 2). The mean percentage hatched egg was significantly higher (p<0.05) in Treatment 5 than others and ranged from 80 to 100% for both halves. Egg hatchability rate in F2 (60) was significantly higher (p<0.05) than F1 (60) except in T5 where it was grossly similar.
.png) Figure 2 Mean percentage Hatched egg of Clarias gariepinus (Error bar = SEM) |
3.3 Effect of supplementation of Vitamin A on eggs and liver retinol of African Catfish (C. gariepinus) brood stock
Effect of Supplementation of Vitamin A on egg retinol concentration of African Catfish (C. gariepinus) Broodstock is presented (Table 5) and the difference between the two halves (Figure 3). The retinol concentration in fish eggs was significantly higher in T5 than others and ranged from 175 to 3884 and 204 and 4022 IU kg-1 for F1(60) and F2(60) respectively. Values for F2 (60) was significantly higher (p<0.05) than F1 (60).
.png) Figure 3 Retinol content in eggs of Clarias gariepinus |
3.4 Effect of supplementation of vitamin A on vitamin A concentration in liver of African Catfish (C. gariepinus) brood stock
No retinol concentration was seen in the fish liver as all the analyses indicated zero concentration.
3.5 Effect of supplementation of vitamin A on Estradiol (E2) concentration in ovaries of African Catfish (C. gariepinus) brood stock
Effect of Supplementation of Vitamin A on E2 concentration of African Catfish (C. gariepinus) Broodstock is presented (Table 5) and the difference between F2 (60) and F1 (60) (Figure 4). The E2 residues significantly (p<0.05) increased with increase concentration and was significantly higher (p<0.05) in F2 (60).
.png) Figure 4 Estradiol concentration in ovaries of Clarias gariepinus |
3.6 Effect of supplementation of Vitamin A on larval survival rate
Effect of Supplementation of Vitamin A on larval survival rate is presented and the difference between F1 (60) and F2 (60) (Figure 5). The larval survival rate ranged from 65.8 to 86.8 and 68.3 to 89.5 percent for F1 (60) and F2 (60) respectively. Treatment 3 to 5 were significantly higher (P<0.05) than others and F2 (60) also significantly higher than F1 (60).
.png) Figure 5 Larval survival rate |
4 Discussion
During the 180 days three phases'culture, the physicochemical parameters were within the optimum range recommended for the culture of freshwater fishes in the tropical region (Okoliegbe, 2020). The ammonia concentration recorded in this study is 0.03 mg L-1. This range is higher than < 0.01 stipulated by FEPA. The relatively high level of ammonia observed in the five Treatments could be attributed to accumulation of un-eaten protein-rich feed and fish wastes. Ammonia is the most abundant form of dissolved inorganic nitrogen. It is used as indicator of organic pollution and becomes toxic to aquatic organisms in concentration of more than 2~5 mg L-1 (Khattaby, 2015).
The dissolved oxygen (DO) ranged from 5.14 mg L-1 and 5.72 mg L-1 across the five Treatments. Ehiagbonare and Ogunrinde (2010) observed a higher range of 9.3 mg L-1~16.2 mg L-1 in concrete and earthen fish ponds analyzed in Edo State, Nigeria. Towers (2014) posited that a minimum constant DO of 4.0 mg L-1 is adequate for optimum yield in aquaculture. These results imply that fishes in all the fifteen ponds analyzed in this study had adequate dissolved oxygen supply. Low DO is caused by small size of pond and eutrophication due to over fertilization with manure. Consistently high DO levels can cause the gas bubble disease while consistently low levels of DO (1.5 mg L-1) reduces the fish feed intake and growth rate. Fish breathe in oxygen for carrying out metabolic activities. DO is also needed for breaking down potentially harmful metabolic wastes into less harmful substances e.g. breaking down ammonia (NH3) into nitrites (NO2) and nitrates (NO3) (Towers, 2014).
The morning temperature range of 23.4 °C~24.4 °C observed in the ponds was within the recommended temperature range for aquaculture. Solomon et al. (2013) observed a wider temperature range of 24 °C~29 °C in fishponds studied in Abuja. Temperature determines the rate of metabolism in aquatic organisms. Temperature affects the solubility of oxygen in water. If the temperature is high, lesser oxygen dissolve at the water-air interface, and dissolved oxygen in the deeper parts of the pond floor further decreases (Ezekiel et al., 2011).
The pH increased with the salinity and alkalinity of the medium. The pH range of 7.21~7.83 recorded in the pond was within WHO limit (6.8~8.5) for optimum aquaculture Low pH values favour the existence of toxic heavy metals in mobile form making the water hazardous for aquaculture (Olatayo, 2014). One of the essential benefits of intensive recirculation structures is the capacity to manipulate the aquatic surroundings and essential physico-chemical parameters to maximize fish fitness and boom fees. Crucial water high-quality parameters that play vital roles in fish production include temperature, pH and concentrations of dissolved oxygen, ammonia etc. Every precise parameter is crucial, but it is the cumulative effect and interrelationship of all of the parameters which have an effect on the health and growth of the fish. The presence of every natural and inorganic material in fish rearing water influences the survival, fitness, increase and widespread well-being of the fish. Wisdom et al. (2013) studied the physicochemical parameters of fish ponds in Abuja, Nigeria and found that there may be a robust correlation among physicochemical parameters and fish mortality. Habitual monitoring of physicochemical parameters helps in early detection of degradation of water fine in order that timely correction measures can be undertaken to avoid stunted boom, disorder outbreaks or fish demise. Detrimental alterations in important parameters like dissolved oxygen, temperature and ammonia can cause fish death and huge losses in aquaculture (Wisdom et al., 2013).
Vitamin A (VA) is an essential dietary nutrient for fish and plays an important role in a range of physiological processes includingvision, reproduction, embryogenesis, growth and differentiation and maintenance of epithelial cells. According to Wang et al. (2014) VA stimulated the maturation of the ovary of starry flounder. The quality of larvae and eggs is a very important parameter in the aquaculture industry, because a large quantity of eggs of optimum quality is necessary to allow further development of the cultured organism. The main parameters considered in the evaluation of progeny quality are egg viability, hatching rate, and survival. However, other markers exist to establish quality during embryo development or in the vitelline larval stage, for example, egg morphometry, oil drop, biochemical composition of yolk reserves, and malformations in larvae.
Regarding the supplementation of vitamin A (retinol palmitate) in the diet of C. gariepinus in this study, the mean percentage fertilized egg was significantly higher in diets 6664RE and 13,328RE than others and ranged from 73 to 98% for both halves. Egg fertilization rate in F2 was significantly higher (P<0.05) than F1. It can therefore be deduced that the optimum level of VA required for best percentage fertilized egg is within the region of 6664 to 13,328 IU kg-1. Mean percentage hatched egg was also significantly higher (P<0.05) in diet 13,328RE than others and ranged from 80 to 100% for both halves. Egg hatchability rate in F2 was significantly higher (P < 0.05) than F1. Similar result was obtained when Wang et al. (2014) fed Platichthysstellatus broodstock with different levels of VA inclusion. The inclusion of this vitamin was observed to slightly improve the percentage of viable eggs, the hatching rate, and level of larval survival. However, higher retinol concentrations were observed in the ovaries of females supplemented with vitamin A (49.7 μg·g-1) compared to those not supplemented (5.1 μg·g-1), as well as darker pigmentation in the skin of broodstock in the supplemented group. According to Furuita et al. (2003); Palace and Werner (2006) addition of VA improved eggs quality and maintain early development in Japanese flounder (Paralichthys olivaceus).
Total retinol contents in eggs increased with increasing levels of dietary VA. No retinol was detected in livers. This disagrees with the findings of Hernandez et al. (2004) who stated that, “Fish fed on diets with 0 and 300 retinol eq kg-1 diet showed a significantly lower content of vitamin A (as a total retinol) in the liver than those in groups fed with higher levels of supplementation. Vitamin A was stored in the liver mainly as retinyl esters. Total retinal content of the eye showed no significant differences among the treatments; however, fish fed with 0 and 300 retinol eq kg-1 of diet had slightly higher concentrations”. Hernandez et al. (2005) also stated that, “Total retinol contents in liver and eyes of juvenile Japanese flounder (Paralichthys olivaceus) increased with increasing levels of dietary VA. No retinol was detected in livers, and significantly lower total retinol content was observed in eyes of fish fed 0 IU kg-1”. There is agreement in these findings though these were different fish species.
The E2 residues in C. gariepinus brood stocks were below the biologically significant level of 10 ng L-1. 17 beta-estradiol (E2) is a female hormone which is known to be one of the strongest estrogenic chemicals in the environment. According to Imai et al. (2005) reproduction in the Java-medaka, especially the male fish could be affected by exposure to E2 concentrations greater than 16 ng L-1. Exogenous estrogen 17 beta-estradiol (E2) has been shown to effectively induce feminization in channel catfish (Wang et al., 2022). Sun et al. (2020) stated that 1ng L-1~10 ng L-1 estradiol (E2) concentration can create feminized properties in male fish; this amount for synthetic estrogen was 0.1 ng L-1. Bhardwaj et al. (2019) reported that estrogens can function as initiators and promoters in cancer progression. Furthermore, a study showed that estrogen or their derivatives have carcinogenic properties on the kidneys, liver, uterus and mammary glands in rat (Kakehashi et al., 2016). Estradiol (E2) residue is one of the considerable endocrine-disrupting compounds and has negative effects on human embryonic and wildlife especially on fish (Amenyogbe et al., 2020). According to Gibson and Saunders (2012), E2 can be accumulated in bile and ovaries and testes of fishes and disrupts the natural activities in fish.
Fortification of broodstock diet with VA also enhanced fry survival. The range of 65.8 to 89.5% in this study is better than 70.19 to 73% reported for Silver Carp (Hypophthalmichthys molitrix) in an unfortified diet. The better fry survival in the second spawning cycle agrees with findings of Umanah (2020) who reported that maternal age has positive impact on the fry survival rate.
5 Concluding Remarks
The results of this study showed that the addition of higher levels of vitamin A to broodstock feed had a significant positive effect on the reproductive performance and larval survival of African catfish (Clarias gariepinus). Specifically, vitamin A supplementation to the levels of 6664 IU/kg and 13328 IU/kg significantly improved egg quality, as evidenced by increased fertilization and hatchability, increased retinol content in eggs, and increased estradiol (E2) concentration in the ovaries. As vitamin A levels increased, larval survival also improved significantly, especially during the second spawning cycle. These results suggest that vitamin A supplementation is essential for improving reproductive success and early larval development in aquaculture, providing a potential strategy for commercial hatcheries to improve productivity.
However, the positive effects observed in this study must be combined with sustained and effective water quality management. Parameters such as dissolved oxygen, ammonia, pH, and temperature are kept within optimal ranges during the experiment, which is essential for maintaining the health and reproductive performance of the broodstock. Regular monitoring and control of these water quality parameters is key to fully realizing the benefits of dietary interventions such as vitamin A supplementation.
Given that higher vitamin A levels are associated with significant improvements in reproductive outcomes and larval survival, it is recommended that fortified broodstock diets with vitamin A levels up to 13 328 IU/kg be used in aquaculture to improve reproductive performance. Further research into the long-term effects of this supplement on offspring and overall fish stock quality will help refine these recommendations and maximize the potential of vitamin A in broodstock management.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Adebayo O.T., 2006., Reproductive performance and heterosis in reciprocal Clarias hybrids between Clarias gariepinus and Clarias anguillaris, Journal Fish, Int., 6(3): 67-70.
Alagawany M., Elnesr S.S., Farag M.R., Tiwari R., Yatoo M.I., Karthik K., Michalak I., amd Dhama K., 2020, Nutritional significance of amino acids., vitamins and minerals as nutraceuticals in poultry production and health-a comprehensive review, Veterinary Quarterly, 41(1): 1-29.
https://doi:10.1080/01652176.2020.1857887
Amar E., Kiron V., Satoh S., and Watanabe T., 2004, Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products, Fish & Shellfish Immunology, 16: 527-537.
https://doi.org/10.1016/j.fsi.2003.09.004.
Amenyogbe E., Chen G., Wang Z., Lu X., Lin M., and Lin A.Y., 2020, A review on sex steroid hormone estrogen receptors in mammals and fish, International Journal of Endocrinology, 2020(1): 5386193.
https://doi.org/10.1155/2020/5386193
Berlinsky D.L., Kenter L.W., Reading B.J., Frederick W., and Goetz F.W., 2020, Chapter 1-regulating reproductive cycles for captive spawning, Fish physiology, Academic Press, 38: 1-52.
https://doi.org/10.1016/bs.fp.2020.09.001
Belton B., Little D., and Sinh L.X., 2010, Pangasius seed quality in Vietnam, Pt 1: 36-38.
Berntssen M.H., Ørnsrud R., Rasinger J., Søfteland L., LockE.J., Kolås K., Moren M., Hylland K., Silva J., Johansen J., and Lie K., 2016, Dietary vitamin A supplementation ameliorates the effects of poly-aromatic hydrocarbons in Atlantic salmon (Salmo salar), Aquat Toxicol, 175: 171-83.
https://doi.org/10.1016/j.aquatox.2016.03.016
Bhardwaj P., AuC.M.C., Benito-Martin A., Ladumor H., Oshchepkova S., Moges R., and Brown K.A., 2019, Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression, The Journal of Steroid Biochemistry and Molecular Biology, 189: 161-179.
https://doi.org/10.1016/j.jsbmb.2019.03.002
Chattopadhyay N.R., 2017, Chapter 4-selective breeding in: induced fish breeding – a practical guide for hatcheries, 61-104.
https://doi.org/10.1016/B978-0-12-801774-6.00004-3
Cuesta A., Ortuño J., Rodriguez A., Esteban M., and Meseguer J., 2002, Changes in some innate defence parameters of seabream (Sparus aurata L.) induced by retinol acetate, Fish & Shellfish Immunology, 13: 279-291.
https://doi.org/10.1006/fsim.2001.0403
Ehiagbonare J.E., and Ogunrinde Y.O., 2010, Physico-chemical analysis of fish pond water in Okada and its environs, Nigeria, African Journal of Biotechnology, 9(36): 5922-5928.
Ezekiel E.N., Hart A.I, and Abowei J.F.N., 2011, The physical and chemical condition of sombreiro river, Niger Delta, Nigeria, Research Journal of Environmental and Earth Sciences, 3(4): 327-340.
Fontagné-Dicharry S., Lataillade E., Surget A., Brèque J., Zambonino-Infante J., and Kaushik S.J., 2010, Effects of dietary vitamin A on broodstock performance., egg quality, early growth and retinoid nuclear receptor expression in rainbow trout (Oncorhynchus mykiss), Aquaculture, 303(1-4): 40-49.
https://doi.org/10.1016/j.aquaculture.2010.03.009
Furuita H., Tanaka H., Yamamoto T., Suzuki N., and Takeuchi T., 2002, Effects of high levels of n-3 HUFA in broodstock diet on egg quality and egg fatty acid composition of Japanese flounder, Paralichthys Olivaceus, Aquaculture, 210(1-4): 323-333.
https://doi.org/10.1016/S0044-8486(01)00855-9
Furuita H., Tanaka H., Yamamoto T., Shiraishi M., and Takeuchi T., 2001, Effects of high dose of vitamin A on reproduction and egg quality of Japanese flounder Paralichthys olivaceus, Fisheries Science, 674: 606-613.
https://doi.org/10.1046/J.1444-2906.2001.00296.X
Furuita H., Tanaka H., Yamamoto T, Suzuki N., and Takeuchi T., 2003, Supplemental effect of vitamin A in diet on the reproductive performance and egg quality of the Japanese flounder Paralichthys olivaceus (T & S), Aquaculture Research, 34(6): 461-468.
https://doi.org/10.1046/j.1365-2109.2003.00831.x
Gliszczyńska-Świgło A., and Rybicka I., 2021, Fast and sensitive method for phosphorus determination in dairy products, J Consum Prot Food Saf, 16: 213-218.
https://doi.org/10.1007/s00003-021-01329-x
Gibson D.A., and Saunders P.T.K., 2012, Estrogen dependent signalling in reproductive tissues – a role for estrogen related receptors, Molecular and Cellular Endocrinology, 348: 361-372.
https://doi.org/10.1016/j.mce.2011.09.026
Hernandez L.H.H., Teshima S.I., Koshio S., Ishikawa M., Tanaka Y., and Alam M.S., 2007, Effects of vitamin a on growth, serum anti-bacterial activity and transaminase activities in the juvenile Japanese flounder, Paralichthys olivaceus, Aquaculture, 262(2-4): 444-450.
https://doi.org/10.1016/j.aquaculture.2006.10.012
Hernandez L.H.H., Teshima S.I., Ishikawa M., Alam S., Koshio S., and Tanaka Y., 2005, Dietary vitamin a requirements of juvenile Japanese flounder Paralichthys olivaceus, Aquaculture Nutrition, 11(1): 3-9.
https://doi:10.1111/j.1365-2095.2004.00317.x
Hernandez L.H.H., Teshima S.I., Ishikawa M., Koshio S., and Tanaka H., 2004, Effects of dietary vitamin a on juvenile red sea breamchrysophrys major, Journal of the World Aquaculture, 35(4): 436-444.
https://doi.org/10.1111/j.1749-7345.2004.tb00108.x
Imai S., Koyama J., and Fujii K., 2005, Effects of 17 beta-estradiol on the reproduction of Java-medaka (Oryzias javanicus), a new test fish species, Marine Pollution Bulletin, 51(8-12): 708-714.
https://doi:10.1016/j.marpolbul.2005.02.018
Izquierdo M.S., Fernández-Palacios H., and Tacon A.G.J., 2001, Effect of broodstock nutrition on reproductive performance of fish, Aquaculture, 97(1-4): 25-42.
https://doi.org/10.1016/S0044-8486(01)00581-6
Kakehashi A., Ishii N., Fujioka M., Doi K., Gi M., and Wanibuchi H., 2016, Ethenol-extracted brazilian propolis exerts protective effects on tumorigenesis in wistar hannover rats, PLoS ONE, 11(7): e0158654.
https://doi.1371/jounal.pone.0158654
Khattaby A.A.M., 2015, Effect of different managements of fish ponds on fish productivity and water quality, Benha University, 2015.
Kienzle E., and Zeyner A., 2010, The development of a metabolizable energy system for horses, Journal of Animal Physiology and Animal Nutrition, 94(6): e231-e240.
https://doi:10.1111/j.1439-0396.2010.01015.x
Kumar A., Kamboj M., and Virender, 2021, A review on photometric methods for the quantitation of vitamin A, Microchemical Journal, 171: 106791.
https://doi.org/10.1016/j.microc.2021.106791
Li X., and Zhai Q., 2020, Spectrophotometric determination of Calcium with Dibromo-p-methylsulfonazo, Journal of Chemistry, 2020(1): 9232385.
https://doi.org/10.1155/2020/9232385
Madhu C., Khrisna K.M., Reddy K.R., Lakshmi P.J., and Kelari E.K., 2017, Estimation of crude fibre content from natural foodstuffs and its laxative activity induced in rats, Int. J. Pharma. Res. Health Sci., 5(3): 1703-1706.
https://doi.org/10.21276/ijprhs.2017.03.04
Maehre H.K., Dalheim L., Edvinsen S.K., Elvevoll E.O., and Jensen I.J., 2018, Protein determination-Methods Matter, Foods, 1: 5.
https://doi:10.3390/foods7010005
Malik J., and Mandal S.C., 2022, Chapter 2-Extraction of herbal biomolecules, Herbal Molecule In Healthcare Application, 2022: 21-46.
https://doi.org/10.1016/B978-0-323-85852-6.00015-9
Mengging L., Wen J.J., Qing C., and Jialin W., 2004, The effect of vitamin a supplementation in broodstock feed on reproductive performance and larval quality inPenaeus chinensis, Aquaculture Nutrition, 10(5): 95-300.
https://doi.org/10.1111/j.1365-2095.2004.00302.x
Mejri S.C., Tremblay R., Audet C., Wills P.S., and Riche M., 2021, Essential fatty acid requirements in tropical and cold-water marine fish larvae and juveniles, Front. Mar. Sci., 8: 680003.
https://doi:10.3389/fmars.2021.680003
Nguyen P.T., Bui T.M., Nguyen T.A., and De Silva S., 2013, Developments in hatchery technology for striped catfish (Pangasianodon hypophthalmus), Technology and Nutrition, Advances In Aquaculture Hatchery Technology, Woodhead Publishing, 2013: 498-518.
https://doi.org/10.1533/9780857097460.3.498
NRC (National Research Council) 2011, Nutrient Requirements of Fish and Shrimp; National Academic Press: Cambridge., MA., USA.
Núñez-Sánchez N., Requena-Domenech F., García-Moya M., Peña-Blanco P., Agüera-Buendía E., and Martínez-Marín A.L., 2019, Predicción del contenido en energía digestible de alimentos para equinos a partir de la composición química proximal, Rev, Científica FVC-LUZ, 29: 126-132.
https://produccioncientificaluz.org/index.php/cientifica/article/view/29599
Okoliegbe I.L., Ariole N., and Okpokwasili G.C., 2020, Physico-chemical properties of concrete pond water used for Clarias gariepinus aquaculture, Nat Sci., 18(7): 48-55.
Olatayo A.A., 2014, Assessment of physico-chemical parameters of waters in ilaje local government area of ondo state, Nigeria, ijfas, International Journal of Fisheries and Aquatic Studies, 1(5): 84-92.
Palace V.P., and Werner J., 2006, Vitamins A and E in the maternal diet influence egg quality and early life stage development in fish: a review, Sci. Mar, 70(S2): 41-57.
Pundir C.S., Nohwal B., and Chaudhary R., 2021, A comprehensive review of methods for determination of l-lysine with detailed description of biosensors, International Journal of Biological Macromolecules, 186: 445-461.
https://doi:10.1016/j.ijbiomac.2021.07.010.
Segura A., Rodriguez-Caro R.C., Graciá E., and Acevedo P., 2021, Differences in reproductive success in young and old females of along-lived species, Animals (Basel), 11(2): 467.
Solomon W.G.O., Olatunde A.A., and Matur B.M., 2013, Some physicochemical parameters of selected fish ponds in gwagwalada and kuje area councils., federal capital territory, Nigeria, Global Advanced Research Journal of Agricultural Science, 2(1): 017-022.
Shabir U., Raja R., and Khan I.A., 2018, Estimation of proximate composition (moisture and ash content) of some economically important fishes of the valley, International Journal Of Advance Research in Science and Engineering, 7(4): 2046-2053.
Sun S.X., Wu J.L., Lv H.B., Zhang H.Y., Zhang J., Limbu S.M., Qiao F., Chen L.Q., Yang Y., Zhang M.L., and Du Z.Y., 2020, Environmental estrogen exposure converts lipid metabolism in male fish to a female pattern mediated by AMPK and mTOR signalling pathways, Journal of Hazardous Materials, 394: 122538.
https://doi.org/10.1016/j.jhazmat2020.122537
Tacon A.G.J., 2000, Broodstock nutrition: Effects of nutrient levels, Global Aquaculture Advocate, 1-5.
https://www.aquaculturealliance.org/
Towers L., 2014, Water quality monitoring and management for catfish pond, The Fish Site, 718(1): 012061.
Udo I.U., Ndome C.B., Ekanem S.B., and Asuquo P.E., 2011, Application of linear programming technique in least-cost ration formulation for African catfish (Clarias gariepinus) in semi-intensive culture system in Nigeria, Journal of Fisheries and Aquatic Science, 6(4): 429-437.
http://doi:10.3923/jfas.2011.429.437
Umanah S.I., 2020, Maternal age influence on fry survival, growth and size variation in clarias gariepinus, Asian Journal of Animal Sciences, 14: 145-152.
Wang W., Tan S., Yang Y., Zhou T., Xing D., Su B., Wang J., Li S., Shang M., Gao D., Dunham R., and Lui Z., 2022, Feminization of channel catfish with 17β-estrogen involves methylation and expression of a specific set of genes independent of the sex determination region, Epigenetics, 17(12): 1820-1837.
https://doi:10.1080/15592294.2022.2086725.
Wang J., Li B., Liu X., Ma J., Wang S., and Zhang L., 2014, Dietary vitamin A, ascorbic acid and α-tocopherol affect the gonad development and reproductive performance of starry flounderPlatichthys stellatusbroodstock, Chin.J.Ocean.Limnol., 32: 326-333.
https://doi.org/10.1007/s00343-014-3162-y
Webster C.D., and Lim C., 2001, Nutrition and fish health, Academic Press, 2003: 671-702.
Wisdom S.G.O., Olatunde A.A., and Matur B.M., 2013, Some physicochemical parameters of selected fish ponds in gwagwalada and kuje area councils, Federal Capital Territory, Nigeria, Global Advanced Research Journal of Agricultural Science, 2(1): 017-022.
Wybraniec S., Michalowski T., and Garcia A.A., 2013, An overview of the Kjeldahl method of nitrogen determination, Part II, Sample preparation., working scale, instrumental finish and quality control, Crit.Rev.Anal.Chem.,43: 224-272.
https://doi.org/10.1080/10408347.2012.751787
Zeyner A., and Kienzle E.A., 2002, Method to estimate digestible energy in horse feed, The Journal of Nutrition, 132(6): 1771S-1773S.
https://doi:10.1093/jn/132.6.1771S
Zou L., Lin H., and Jiang L., 2007, Determination of β-Estradiol Residues in Fish/Shellfish Muscle by gas chromatography-mass spectrometry, Chinese Journal of Analytical Chemistry, 35(7): 983-987.
https://doi.org/10.1016/S1872-2040(07)60063-2
.png)
. PDF(1436KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Okure G.P.
. Imefon Udo
. Afia O.E.
Related articles
. Nutrition
. Feed
. Growth
. Aquaculture
. Health
Tools
. Email to a friend
. Post a comment


